The estimated reading time for this post is 48 Minutes
A Meta Analysis of Septal Occlusion Devices compared against Cardiac Surgery for the closure of Ostium Secundum type Atrial Septal Defects: Safety, Efficacy and Costs
1. Abstract
Background: Ostium secundum type Atrial Septal Defect (OsASD) is a heart defect, usually present at birth, which makes up approximately 10% of all cases of congenital defects. There are currently two primary methods of closure through septal occlusion devices or surgery. They both produce excellent results, however, neither has been established as the best method of closure.
Objectives: To determine the safety, efficacy and cost of the two procedures through evaluating outcome measures such as rate of complications, closure rate, length of hospital stay and the cost of the procedure.
Methods: The databases Medline, Pubmed, Embase and EBSCO were all searched using the search terms ‘atrial septal defect’ and ‘ostium secundum’, with either of the following: ‘comparative study’, ‘retrospective study’, ‘comparison’, ‘versus’, yielding 391 studies. After applying the inclusion and exclusion criteria, 9 studies were identified as suitable for statistical analysis.
Results: The results showed that the rate of complications and length of hospital stay were significantly in favour of the device group, producing fewer complications and a shorter length of stay in hospital. The surgical group was shown to undergo complete closure at a significantly higher rate than the device group and produced lower costs in two of the three studies analysed.
Conclusion: In order to comprehensively assess each procedure, groups of individuals with similar physiological baselines and characteristics should be pooled together and treated, with subgroup statistical analysis as well as an overall comparison.
Table of Contents
Click to expand Table of Contents
1. Abstract
2. Introduction
2.1 Atrial Septal Defects
2.2 Aims and Objectives
2.3 PICO
3. Background
3.1 Embryology of the Heart
3.1.1 Days 0-7
3.1.2 Implantation in the uterus
3.1.3 Gastrulation and Neurulation
3.1.4 Transverse folding of the embryo
3.1.5 Formation of the primitive heart
3.1.6 Separation of the heart chambers
3.1.7 After Birth
3.2 Causes of ASD
3.2.1 Genes
3.2.5 Environmental Factors
3.3 Complications
3.3.1 Eisenmenger’s Syndrome
3.3.2 Paradoxical Embolism
3.4 Symptoms
3.5 Diagnosis
3.5.1 Auscultation
3.5.2 Echocardiography
3.5.3 Electrocardiogram (ECG)
3.5.4 Chest X-ray
3.6 Treatment
4. Methods
4.1 Search Method
4.2 Inclusion Criteria and Justification
4.3 Data Extraction
4.4 Statistical analysis
4.5 Outcome measures
4.6 PRISMA Flowchart
5. Results
5.1 Objective 1
5.2 Objective 2
5.3 Objective 3
5.3.1 Length of hospital stay
5.3.2 Cost of Procedure
6. Discussion
6.1 Objective 1
6.2 Objective 2
6.3 Objective 3
6.3.1 Length of hospital stay
6.3.2 Cost of procedure
6.4 Limitations
7. Recommendation and Conclusion
References
2. Introduction
2.1 Atrial Septal Defects
An atrial septal defect (ASD) is a congenital defect defined as a patent hiatus found between the two collecting chambers of the heart, the right and left atria, due to a lack of growth in the inter-atrial wall (Table 1)[1]. It is common making up approximately 10% of all congenital heart defect cases, occurring in approximately 2 per 1000 live births, although this figure may be higher as it is significantly under-diagnosed [2]. It occurs in more often in women than men, at a ratio of roughly 2:1[3].
| Type of ASD | Description |
| Patent Foramen Ovale
|
|
| Ostium Primum
|
|
| Sinus Venosus
|
|
| Unroofed Coronary Sinus |
|
Table 1: A table showing the different types of atrial septal defects that occur and their characteristics.
The ostium secundum type ASD (OsASD) is the most common, occurring in over 70% of cases and is located in the centre of the inter-atrial wall involving the fossa ovalis[8]. Patients who are diagnosed have to be assessed to decide whether intervention is necessary or not. Often, children under the age of five have the potential for the defect to close naturally, however if it does not, closure is attempted.
Many patients remain undiagnosed until adulthood due to their lack of symptoms, leaving them at risk of a number of potentially fatal complications such as Eisenmenger syndrome, heart failure, atrial fibrillation and paradoxical emboli, highlighting the need for an early diagnosis and treatment to be undertaken[9].
The two primary interventions of treatment is the closure of the hiatus through cardiac surgery or a less invasive approach through the use of septal occluders. Cardiac surgery had been the gold standard of treatment for over 60 years with experienced surgeons boasting a low mortality and high closure rates[10]. However, since King performed the first non-invasive surgery to treat this condition in 1976[11], there has been a drive towards a lower risk and less invasive surgery to improve outcomes for patients. When septal occlusion devices became popular in the 1990s, the potential of treating elderly patients has become more feasible as the procedure is minimally invasive, providing less risk to the patient[12]. This push to be less invasive has placed a new emphasis on post-operative quality of life and other factors such as aesthetics or financial benefit[13].
Cardiac surgery is the generic term used when surgically operating on the heart or great vessels. Open-heart surgery is the most established procedure and access is gained to the heart via a median sternotomy (Figure 1[14]). As medical technology has advanced, different methods and procedures have been adapted depending on the need in order to produce less invasive approaches with the same level of accessibility to the heart, for example, the anterolateral thoracotomy (Figure 2[15]).
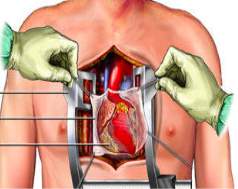
Figure 1: A diagram illustrating how a median sternotomy is performed. Access is gained to the chest cavity via the sternum in order to give surgeons full access to the heart.
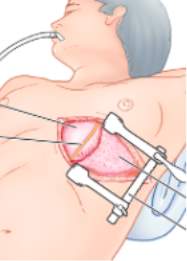
Figure 2: A diagram illustrating an anterolateral thoracotomy. Access is gained to the chest cavity via the rib cage in order to reach the heart.
Septal occluder devices on the other hand, gain access to the heart via the peripheral circulation. The device is placed on the end of a thin tube known as a catheter and placed into the body through the femoral vein found in the leg. The catheter is passed up through the body to the heart and inflated, closing the defect. This encourages new tissue to grow and form a new inter-atrial septum (Figure 3[16]).
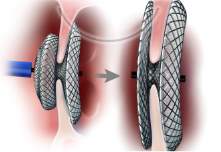
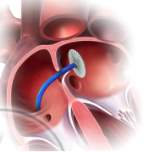

Figure 3: A diagram illustrating the process of inserting a septal occluder into the defect. In the diagram on the left, the catheter can be seen inserted through the defect. On the right, the diagram shows the catheter being placed into the defect and then detached, leaving the occluder in place.
Both of these procedures are still commonly used today, however, there is not sufficient evidence in literature to imply whether one produces better results than the other, despite OsASD being one of the most common congenital defects. The purpose of this investigation is to collate the data from similar studies that compare each intervention and identify which of the interventions produces better outcome measures. This evidence-based medicine approach would be beneficial to clinicians, allowing them to make an informed decision based on the most recent data to decide what is best for their patients, providing further reassurance for patients[17].
2.2 Aims and Objectives
The aim of this study is to determine which of the two procedures is best- closure through cardiac surgery or septal occluders- by evaluating the effectiveness, safety and cost of septal occluders and cardiac surgery in the treatment of the ostium secundum type atrial septal defects in both adults and children. The primary objectives are:
- Assess which intervention is has a higher efficacy for by comparing outcome measure: closure rate
- Assess which intervention is safer for patients to undergo by comparing the outcome measure: rate of complications
- Assess which intervention is cheaper for patients to undergo by comparing outcome measures: length of hospital stay and procedural cost
2.3 PICO
Population – Adults (<65 years old) and children suffering from an ostium secundum type atrial septal defect
Intervention – Transcatheter closure using a septal occluder, the current standard treatment for ostium secundum atrial septal defects
Comparison – Cardiac surgery through a median sternotomy, anterolateral thoracotomy or minimally invasive surgery
Outcome – To assess and compare whether the use of a septal occluder is statistically safer and has a higher efficacy than the cardiac procedures performed. Similarly, the financial viability of each procedure will be statistically analysed. This will be done through the following outcome measures: rate of complications, closure rate, length of hospital stay and cost.
3. Background
3.1 Embryology of the Heart
3.1.1 Days 0-7
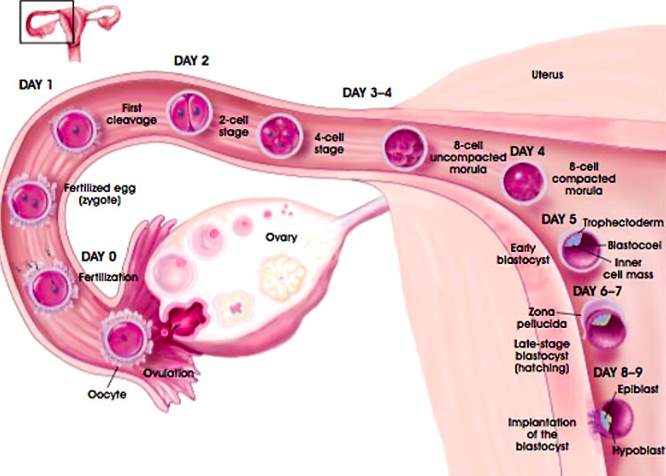
| Figure 4[18]: Fertilization begins when the sperm cells penetrate the ovum forming a zygote. The zygote contains all the necessary genetic information to begin the process of embryogenesis, beginning in the fallopian tube and travelling towards the uterus during the pre-implantation period. Throughout this stage, the embryo undergoes mitotic divisions without growing, receiving nourishment through diffusion from uterine secretions and forming a blastocyst. |
3.1.2 Implantation in the uterus
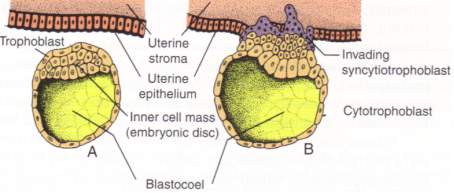
| Figure 5[19]: When the blastocyst makes contact with the lining of the endometrial cavity in the uterus, trophoblast cells fuse with the endometrium forming large multinucleated cells called syncytiotrophoblasts. This occurs at approximately day 8. |
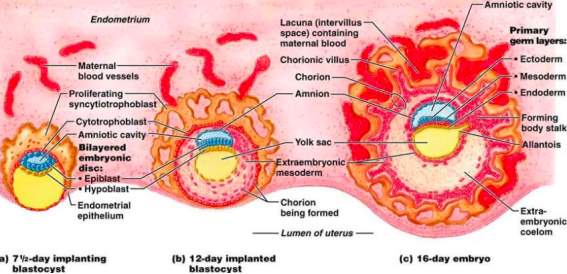
Figure 6[20]: The maternal blood vessels remain in close proximity to the uterine blood vessels, allowing for the diffusion of nutrients into the foetal blood and the removal of waste products into the maternal blood. As the embryo increases in size through embryogenic processes such as blastulation, gastrulation and neuralation, diffusion is no longer able to maintain a sufficient level of exchange to sustain the embryo. It is because of this that the heart is the first organ to form, beginning at approximately 17 days after fertilization[21].
3.1.3 Gastrulation and Neurulation
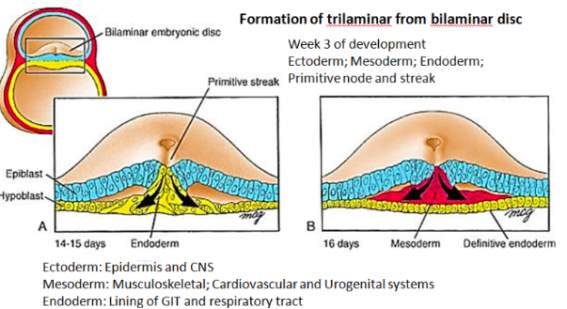
Figure 7[22]: Towards the end of the second week of embryological development, the process of gastrulation has begun. It leads to the formation of three distinct germ layers from which different body systems can be derived.
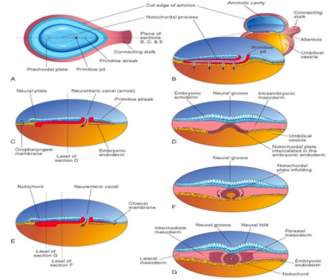
Figure 8[23]: During this time key processes occur which lead to the formation of the embryonic heart. Initially, specialised cells within the mesoderm, called the chordamesoderm, differentiate to form the notochord. The notochord is found ventral to the ectoderm, where it induces the process of neurulation, leading initially to the formation of the neural plate. This leads to the invagination of the ectoderm, leading to the formation of a neural groove with neural folds.
3.1.4 Transverse folding of the embryo
The mesoderm at this stage is split into three areas that form different structures; the paraxial, the intermediate and the lateral plate mesoderm. This slight folding of the neural plate leads to the formation of further divisions within the mesoderm; the paraxial mesoderm separates into somites and the lateral plate mesoderm into the somatic and the splanchnic mesoderm. During this, cells from the lateral sides of the primitive streak in the endoderm travel to the splanchnic mesoderm layer where it develops into the primary heart field.
As the neural plate continues to fold, the lateral plate mesoderm surrounds the connection between the endodermal gut tube and the yolk sac, known as the vitelline duct. Within the splanchnic mesoderm, blood islands and cardiac myoblasts form a pair of mesodermal endothelial tubes and mesenchymal dorsal aortae (Figure 9). This occurs at approximately 17 days after fertilization.
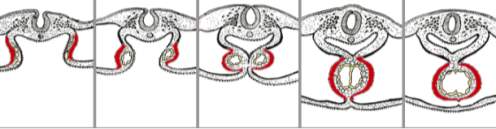
Figure 9[24]: The splanchnic mesoderm can be seen in red. It migrates as transverse folding occurs, surrounding the primitive endocdermal gut tube.
3.1.5 Formation of the primitive heart
By day 22, the surface ectoderm has expanded around the endoderm and mesoderm in a process called transverse folding, as have the cephalic and caudal ends of the embryo, known as longitudinal folding. The amniotic cavity eventually surrounds the entire embryo, with the exception of the yolk sac, playing a key role in the blood supply to the early embryo. When this transverse folding occurs, the splanchnic mesoderm surrounds the gut tube whilst the somatic mesoderm forms part of the body wall[25]. The cavity between these two parts of the mesoderm are known as the intraembryonic coelom. This also leads to the partial fusion of the endometrial tubes forming the basis of a heart (Figure 10).
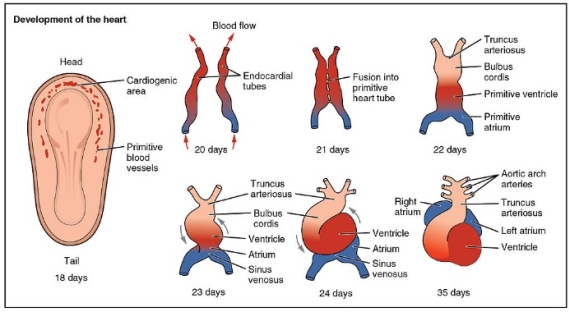
Figure 10[26]: The transformation of the primitive heart from endocardial tubes and it’s development from day 20 to day 35.
At approximately day 23, the heart tube will start beating providing circulation to the embryo, anchored at the cephalic end by the arterial trunks and caudally by extensive venous channels. The five sections of this primitive heart tube go onto develop into different parts of the heart:
- Truncus arteriosus- forms the aorta and pulmonary trunk
- Bulbus cordis- forms the right ventricle
- Primitive ventricle- forms the left ventricle
- Primitive atrium- partitioned to form right and left atria
- Sinus venosus- forms part of the right atrium- the smooth part. Also forms coronary artery and the sinoatrial node[27]
The heart subsequently loops rightwards[28] and rapidly grows through cell proliferation and the recruitment of additional cells. These additional cells are added to both ends of the heart tube and originate from the secondary heart field, found in an area caudal to the outflow tract[29]. The secondary heart field was initially part of the primary heart field, originating from the splanchnic mesodermal cells and the cells primarily contribute to the outflow tract, the right ventricle and a large portion of the atria. This is particularly significant as any defects in secondary heart field can lead to problems in developing heart[30].
The heart undergoes a number of folds, moving the caudal sinus venosus and primitive atria behind and above the primitive ventricles. The primitive ventricles continues to grow in size and length, forming a primitive heart where the left ventricle lies to the left of the interventricular groove and the bulbus cordis region communicates with the truncus arteriosus. At this stage there are no valvular structures or septa separating the early chambers and so blood travels in only one direction27.
3.1.6 Separation of the heart chambers
Throughout the fourth and fifth weeks of embryo development, three septa are formed between the chambers of the heart: the atrioventricular, the interventricular and the interatrial. The inner layer of the heart will form the endocardium and the outer layer will form the myocardium. In between exists a cardiac jelly where endothelial cells travel into and form mesenchymal cells in process known as Endothelial-Mesenchymal transformation[31]. This is significant in the atrioventricular septum where an inward growth of mesenchymal cells go on to form the endocardial cushions. These fuse forming the atrioventricular septum that separates the atrioventricular canals into two, and leads to the formation of bicuspid and tricuspid valves[32] (Figure 11).
The interventricular wall between the ventricles grows from the bulbo-ventricular apex of the heart. This grows upwards but does not fuse with the atrioventricular septum until approximately week seven, leaving the interventricular foramen[33]. Due to the structure of the heart, the lower part of the septum separates the two ventricles whilst the upper part separates the right atrium and left ventricle (Figure 11).
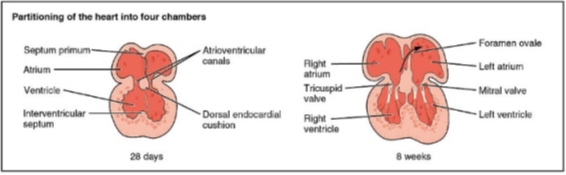
Figure 1126: This diagram highlights the stages in which the septa of the heart develop. All develop at the same time with the dorsal endocardial cushion growing first and acting as a point of growth for the remaining septa.
The interatrial septum develops form three different sources in stages. The septum primum initially grows downwards from the roof of the atria whilst the intermedium septum grows upwards from the atrioventricular canal. Between these two septa, a crescent shaped hiatus known as the ostium primum is formed. Over time, the septum primum grows downwards whilst the septum intermedium grows upwards leading to the closure of the ostium primum. However, blood passing between the atria must persist and thus the holes that are present in the upper part of the primum septum fuse to form a new hiatus called the ostium secundum25 (Figure 12).
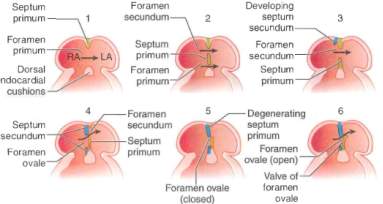
Figure 12[34]: Stages of atrial septum development in the embryo.
Growing downwards and to the right of the septum primum, from the upper part of the atria is a thicker wall called the septum secundum. The anterior part of the septum secundum is able to fuse with the septum intermedium, however the posterior part is not. This gap is closed by the fusion of the left venous valve and the septum spurium. The hole formed between the two atria due to the gap between the septum primum and secundum is known as the foramen ovale[35].
3.1.7 After Birth
From approximately the eight week of embryonic development, the heart has fully formed structurally. Structures like the foramen ovale and ductus arteriosus play a key role in the circulatory system. Oxygenated blood enters foetal circulation from the umbilical cord via the venous system and thus, blood entering the right atrium and would continue onto the pulmonary system. However, in the foetus, the lungs are not operational and thus blood would have to enter systemic circulation via the pulmonary system. In order to avoid this and maintain high levels of oxygenated blood in the systemic circulation, the lungs are bypassed through two mechanisms[36] (Figure 13).
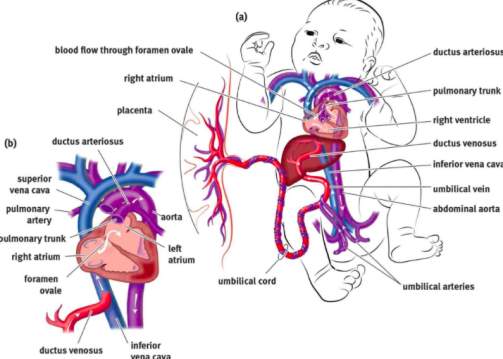
Figure 13[37]: The foramen ovale acts as a valve between the right and left atrium. As pressure in the right atrium is higher than the left, the oxygenated blood is shunted to the left atrium where it mixes with blood from the pulmonary system. This blood is then sent to systemic circulation including the vessels coming off the aortic arch. The ductus arteriosus is connecting artery from the pulmonary artery to the descending aorta, providing oxygenated blood directly to the systemic circulation.
3.2 Causes of ASD
OsASD is primarily due to one of two reasons: either the cephalic portion of the septum secundum undergoes excess cell death or the ostium secundum is too large the septum secundum is unable to close it1. The reasons as to why these variations occur is unknown, however, it is believed that most are due to a complex interplay of genetic and environmental factors.
3.2.1 Genes
Over the last decade or so, advances in genetic technology have allowed researchers to identify and map mutations that interfere with the normal develop of the heart. Through this, a number of conditions and genes have been identified as increasing the risk of development of atrial septal defects:
3.2.2 NKX2.5 and GATA4
Both NKX2.5 and GATA4 have been strongly associated with the development of OsASD. NKX2.5 is a homebox gene that plays a key role in the differentiation of tissues. In the early embryo, it has shown to play a key role in cardiac mesoderm cells, particularly in the atrial chambers[38]. GATA4 is a gene that encodes transcription factors that regulate cardiac genes. It has shown to play a key role in the embryonic heart where it encourages cardiac morphongenesis and cardiomyocte survival and plays a role in the development of non-syndromic ASD[39]. A study conducted by Sarkozy et al, 2005 concluded that there was a strong association with these genes and ASD and recommended genetic counseling alongside normal treatment for patients with a family history[40].
3.2.3 Ebstein’s Anomaly
Ebstein’s Anomaly is characterized by the improper development of the tricuspid valve. Because of this, the pressure in the pulmonary circulation is dramatically increased, causing any communication between the atria to remain open after birth. This is found to occur in over half of patients with Ebstein’s anomaly[41].
3.2.4 Syndromes
| Condition | Description | Genetics |
| Halt Oram syndrome
|
Autosomal disorder causing problems in bones. The syndrome is characterized by a lack of radial bones, atrial septal defects and heart block[42]. | TBX5 shown to reduce the expression of MYH6 (important protein expressed in cardiac atria). Also disrupts interactions between NKX2.5 and GATA4[43]. |
| Lutembacher’s syndrome
|
Characterised by ASD with mitral valves stenosis[44]. | Currently unknown. |
| Noonan Syndrome | Autosomal dominant disorder and considered as male Turner Syndrome. Wide array of disorders associated with it including ASD[45]. | PTPN11 and SOS1 mutations, which play a key role in the early embryonic processes such as differentiation, division and migration43. |
| Down syndrome | Genetic; causes characteristic appearance and learning disabilities. 40%-60% of those with Down’s have an ASD[46]. | Trisomy of Chromosome 2144. |
Table 2: A table showing the characteristics and genetic association of syndromes shown to be associated with ASD.
3.2.5 Environmental Factors
Environmental factors do not play a significant role in the development of ASD, but is believed to account for 2% of cases. Foetal alcohol syndrome has shown to influence the development of the heart with both ASD and ventricular defects common. This is because alcohol consumed by the mother can cross the placenta and affect the developing embryo. The incidence of ASD in babies with foetal alcohol syndrome is over 60%, however there is no conclusive evidence to suggest the precise mechanism as to why this is[47].
3.3 Complications
3.3.1 Eisenmenger’s Syndrome
The right atrium functions as a filling chamber for the venous supply of the systemic circulation, and thus has a low pressure of between 2 and 6 mmHg. The left atrium receives blood from the pulmonary circulation via the pulmonary veins and has a slightly higher pressure between 6 and 12 mmHg[48]. This difference in pressure is significant in patients with OsASD as it allows for a left to right shunt to be formed between the atria, leading oxygenated blood to re-enter the pulmonary system. This increase in pulmonary flow leads to an increase in the pulmonary artery vascular resistance leading to pulmonary hypertension8.
Eisenmenger’s syndrome is the pulmonary hypertension that eventually leads to the reversal of the left to right shunt, due to an increase in the right atrial pressure. This increase in pulmonary pressure can lead to a number of physiological changes. The irreversible damage to the pulmonary capillaries in the lungs leads to a decrease in oxygen saturation. This in turn leads an increase in the erythropoietin levels released from the kidneys and thus increases the overall pressure and viscosity of the blood in the systemic circulation. These physiological changes lead to a number of side effects, including clubbing of the fingers, cyanosis, heart failure and stroke[49].
Eisenmenger’s syndrome is only curable through a lung transplant and surgical treatment to the heart. However, there are a number of medications available to reduce the strain on the heart and lungs such as endothelin receptor antagonists to increase vessel dilation or blood thinning agents to reduce the likelihood of stroke[50].
3.3.2 Paradoxical Embolism
This is nonspecific and occurs when a thrombosis that developed in the venous circulation enters into the systemic circulation via the right to left shunt[51]. It usually develops in the lower extremeties of the body such as the leg or pelvis. They consist of a number of coagulation factors such as platelets and fibrin, as well as red blood cells and can originate from fat, air, tumour or fluid. There are a number of risk factors associated with this including pregnancy, trauma, surgery or cancer and can increase the chances of developing a pulmonary embolism or stroke [52].
3.4 Symptoms
Table 3: A table showing the symptoms seen clinically in ASD.
| Symptoms | Description |
| Shortness of breath | Due to a lack of oxygenated blood reaching the systemic circulation. This can be seen particularly when exercising where a rapid rate of breathing is trying to compensate the lack of oxygenated blood reaching the body8 |
| Fatigue | A lack of energy due to congested circulation to the peripheral muscles, giving a feeling of weakness and fatigue |
| Frequent chest infections | Could be due to a lack of efficient exchange between the oxygen and the blood in the lungs |
| Heart palpitations | Due to dilating of the right atrium, leading to irregularity of AV node stimulation27 |
| Decreased rate of growth | Lack of energy needed to grow and thrive |
3.5 Diagnosis
3.5.1 Auscultation
Auscultation forms an integral part of diagnosing ASD, as the medical practitioner is able to listen to the chest using a stethoscope. In a patient with ASD, the changes to the normal heart sounds can be indicative of an ASD. Typically, sounds indicative of a dilated pulmonary artery can be heard alongside splits in the S1 and S2 heart sound (Figure 14). In addition to this, forceful right ventricular contraction may be heard with a delayed closure of the tricuspid valves[53].
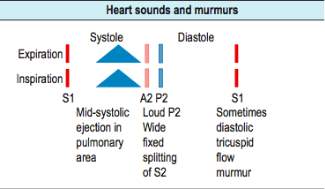
Figure 148: A clinicians guide to heart sounds and what to identify during an auscultation.
3.5.2 Echocardiography
An echocardiogram (ECHO) is a non-invasive test that uses ultrasound to assess the anatomy and physiology of the heart. It is able to do monitor blood flow and detect any structural changes such as right atrial enlargement. There are several different ways to perform an ‘echo’ including:
- Trans-thoracic ECHO- standard method of placing probe on bare chest
- Trans-oesophageal ECHO- small probe swallowed by patient, remaining in the oesophagus and giving varied view of the heart. This is commonly used in the treatment of ASD (See 3.6)
- Intra-cardiac ECHO- scan from within the heart
- Stress ECHO- ECHO performed whilst patient is under exertion[54]
3.5.3 Electrocardiogram (ECG)
In an ECG, a right bundle branch block can be seen due to right ventricular pressure and overload. In addition to this, an enlarged P-wave and a slightly extended P-R can be seen[55].
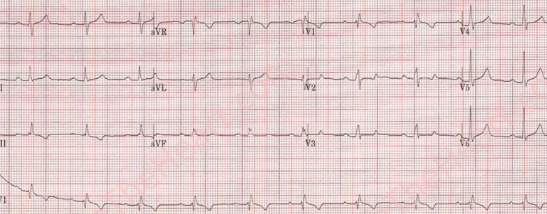
Figure 15[56]: An ECG from a patient with an OsASD
3.5.4 Chest X-ray
Chest X-ray in a patient with ASD shows an enlarged pulmonary artery and excess blood in the lungs as well as a small aortic knuckle and progressive atrial enlargement[57].
3.6 Treatment
In children, closure is recommended regardless of whether they are symptomatic or not in order to avoid any future complications of living with an ASD. In adults, those who are symptomatic or have a pulmonary to systemic blood flow ratio of greater than 1.5:1 are recommended surgery57.
4. Methods
4.1 Search Method
In order to identify the appropriate studies, a number of electronic databases were used in January 2017, including: MEDLINE, PUBMED, EMBASE and EBSCO. As this review is attempting to compare treatments, the key terms used as search terms were ‘atrial septal defect’ and ‘ostium secundum’ were followed by either of the following: ‘comparative study’, ‘retrospective study’, ‘comparison’, ‘versus’. The same search method was applied to each database and for each study. From 391 studies, 382 were discarded as they did not meet the inclusion criteria (Table 3).
4.2 Inclusion Criteria and Justification
| Inclusion | Justification |
| Must be an ostium secundum type defect. | The specific type of ASD I am analysing |
| Must be comparative study. | Must have data on both treatment options |
| Must be looking at defect repair in humans. | Relevant to my topic |
| Must be retrospective study. | Cannot be prospective as had to have produced data for interpretation |
| Studies must be in English. | So that I am able to interpret the data |
| Studies must have been published after January 2000. | To provide up to date information |
| Must have greater than 20 sample size | In order to reduce effects of outliers in the data |
| Study outcome measures must be one of the following:
|
As these are the specific outcome measures I am comparing |
Table 3: The inclusion and exclusion criteria used to determine whether a study was suitable for analysis and the justification for choosing it.
4.3 Data Extraction
Variables extracted from each data included the followings: author name, year of publication, type of treatment delivered and outcome measure. Studies were further assessed to determine whether they meet the criteria to undergo meta- analysis. Additionally, details of each study were extracted such as number of participants and participant’s gender and age.
4.4 Statistical analysis
Statistical analysis for each outcome measure was done through software called Review Manager 5.3. Forest plots were created for each outcome measure with the left hand side of the graph favouring the use of septal occluders and the right hand side favouring the use of cardiac surgery.
For the dichotomous data such as rate of complications and success of surgery for the safety and efficacy of each intervention, the relative risk difference was used to a 95% confidence interval as the difference between two groups are being compared to outcome measures. The method of statistical analysis was the Mantel-Haenszel test and the fixed effect analysis model was applied to the forest plot.
For the continuous data such as length of hospital stay and cost of each procedure for the cost outcome measure, the standard mean difference was used to a 95% confidence interval, and thus the inverse variance was used as a means for statistical analysis.
4.5 Outcome measures
Objective 1: The efficacy of each procedure through the recording of the success rate. Success was defined as the complete closure of the defect during surgery and thus, incomplete closure is defined as failure. As such, in the forest plots, rather than plotting the successful closures as events, plotting the failure of the procedure was more appropriate as failure implies a higher risk associated with the procedure. This yielded forest plots that were able to undergo statistical analysis.
Objective 2: The safety of each procedure through the recording of postoperative complications, both major and minor. When creating the forest plots, the recorded rate of complications was taken directly from the study and input into the table. The collective number of complications that occurred within the study population was logged under the ‘events’ column, whilst the total number of participants was written under the ‘total’ column. Only a single study did not include the total number of complications and thus had to be excluded.
Objective 3: The cost for each procedure was measured through two outcome measures: length of hospital stay and cost of procedure. The length of hospital stay plays a key role as each night in hospital adds to the cost of care to the patient and thus carries significant financial implications. The mean and standard deviation for both outcome measures was necessary to gain a standard mean difference in order to compare the effect size of the outcome measures.
4.6 PRISMA Flowchart
EMBASE
(n=139)
Medline
(n=43)
Pubmed
(n=147)
EBSCO
(n=62)




4.7 Table of Studies (Below)

Records identified from database (n=391)
(n= 1046)
293 studies removed:
- 123 duplicates
- 170 as publication date preceded 2000.

Abstracts screened
(n=98)
83 studies excluded after screening for relevance.

Full text articles assessed for eligibility (n=15)
6 studies removed:
- 2 studies did not meet exclusion criteria
- 4 studies did not meet inclusion criteria

Articles identified
(n=9)
Figure 16: A flowchart to show the progression of searches and the stages in which studies were excluded.
Table 4 (Below): A compilation of studies with all of the data associated with them. ASO- Amplatzer Septal Occluder. CS/SF- CardioSeal StarFlex.
| Study | Number of participants | Male:Female participants | Mean age ± SD (years) | Septal Occluder Device | Surgical technique(s) | Outcomes | |||
| SO | S | SO | S | SO | S | ||||
| Bettencourt et al, 2003[58]
|
38 | 25 | 12:26 | 10:15 | 40 ± NA | 38 ± NA | ASO | Median sternotomy | Closure rate, complications, |
| Butera et al, 2006[59] | 751 | 533 | 336:415 | 124:393 | 29 ± 19.8 | 22.4 ± 18.9 | ASO, CS/SF | Midline sternotomy,ministernotomy,and right lateral thoracotomy | Closure rate, complications, length of hospital stay |
| Hoashi et al, 2015[60] | 709 | 317 | 237:472 | 130:182 | 28± 10 | 24± 16 | ASO | Median sternotomy | Closure rate, complications |
| Kim et al, 2002[61] | 48 | 32 | 31:17 | 22:10 | 37.9±23 | 19.7±19 | ASO | Median sternotomy | Closure rate, complications, length of hospital stay, costs |
| O ‘Byrne et al, 2015[62] | 155 | 89 | 52:103 | 44:45 | 3 ± NA | 5 ± NA | Not specified | Open heart surgery | Costs |
| P. Růžička et al, 2005[63] | 32 | 39 | NA | NA | NA | NA | ASO | Surgical treatment | Closure rate, complications, length of hospital stay, cost |
| Schneeberger et al, 2016[64] | 169 | 95 | 41:128 | 31:64 | 49.6 ± 15.7 | 38.3 ± 12.7 | ASO | Right antero-lateral mini- thoracotomy | Closure rate, complications |
| Suchon et al, 2009[65] | 52 | 45 | 19:33 | 17:28 | 38.9±14.7 | 42.4±13.0 | ASO | Median sternotomy | Closure rate, complications, length of hospital stay |
| Vida et al, 2006[66] | 28 | 83 | 19:9 | 27:56 | 7.14±5.5 | 18.3±15.5 | ASO | Median sternotomy | Closure rate, complications, length of hospital stay, costs |
5. Results
After the removal of duplicates and applying the inclusion and exclusion criteria, 9 studies were identified as suitable for undergoing quantitative analysis. A total of 3240 individuals with an ostium secundum ASD were treated in these studies with 2030 patients undergoing defect closure with a septal occluder, and a further 1210 patients undergoing cardiac surgery.
5.1 Objective 1
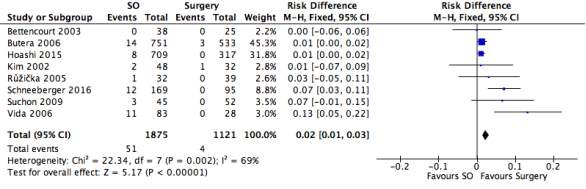
Figure 17: A forest plot illustrating the efficacy of septal occlusion devices compared to surgery through rate of unsuccessful closure.
In order to determine the rate of closure, nine studies were assessed, with 2030 septal occluder patients and 1210 surgical patients. The results showed that fifty-five septal occlusion procedures were unsuccessful in closing the defect whilst only four were unsuccessful from the surgical group. The black diamond on the forest plot represents the average difference in risk found in the eight studies, giving a meta-analytic summary of the data from the studies. It revealed that the overall Risk Difference (RD) was (95% CI): 5.05 [2.27, 11.22], significantly in favour of the surgical procedure. The heterogeneity of the forest plot was high with an I2 value of 69% and a Chi2 value of 22.34.
5.2 Objective 2
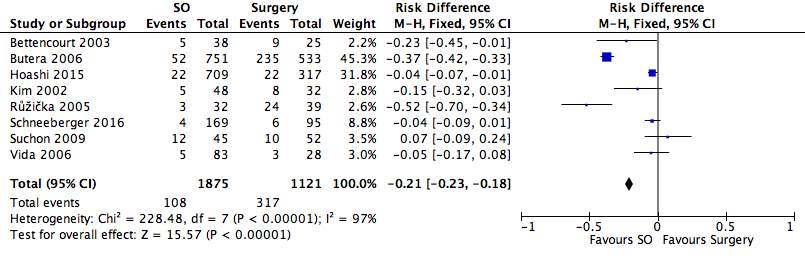
Figure 18: A forest plot illustrating the safety of septal occlusion devices compared to surgery through rate of complications
Of the nine studies found, eight used rate of complications as an indicator of safety. Of 1875 septal occluder patients, 108 complications were documented, whilst in the surgical group, 317 complications occurred in the cohort of 1121 patients. The results showed that the incidence of undergoing complications post-treatment is higher in the surgery group, as supported by the positioning of the black diamond in the forest plot. The overall RD to a 95% confidence interval (95% CI): -0.21 [-0.23, -0.18], which is significantly in favour of the septal occluder group. The heterogeneity of the forest plot was high with an I2 value of 97% and a Chi2 value of 228.48.
5.3 Objective 3
5.3.1 Length of hospital stay
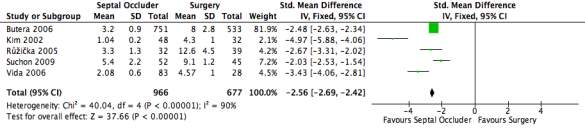
Figure 19: A forest plot illustrating the length of hospital stay of septal occlusion devices group compared to the surgery group, measured in number of days.
This outcome measure was recorded by five of the nine studies used with 966 patients in septal occluder group and 677 patients in the cardiac surgery group. Using the standard mean difference, the mean and standard deviation could be plotted onto the forest plot and compared between the two groups. Although two further studies measured the length of hospital stay, one did not include the standard deviation whilst the other only documented the data for the surgical group. The average length of stay for the septal occluder group was 3.12 whereas the average for the surgical group was 8.02. The standard mean difference was illustrated as a black diamond to a 95% CI: -2.56 [-2.69, -2.42], in favour of the septal occluder group. The heterogeneity of the forest plot was high with an I2 value of 90% and a Chi2 value of 40.04.
5.3.2 Cost of Procedure
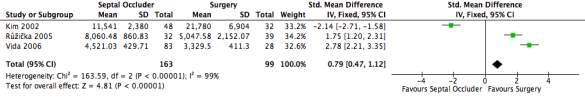
Figure 20: A forest plot illustrating the cost of procedure of septal occlusion devices compared to surgery in USD.
Measuring the cost of each procedure was another outcome measure looking at the financial viability. Of the 163 patients in the septal occluder group, the average cost of the procedure was $8,040.84 whilst the 99 patients in the surgical group had an average cost of $10,0052.36. Several other studies included costs of the procedures, however did not include the mean cost or the standard deviation. The standard mean difference to a 95% CI was 0.79 [0.47, 1.12], favouring the surgical group. The heterogeneity of the forest plot was high with an I2 value of 99% and a Chi2 value of 163.59.
6. Discussion
6.1 Objective 1
Figure 2 shows data from all nine studies where the closure rate for each procedure has been assessed. Due to the nature of forest plots, the outcome measure was not strictly the closure rate; rather it was the number of times in which complete closure did not occur, which were documented as ‘events’. Although all the studies’ RD favoured surgery, the confidence intervals crossed the line of no effect, indicating that the results are not statistically significant. Despite this, the black diamond sits at 5.05 [2.27, 11.22], on the right hand side of the forest plot and not touching the line of no effect. Although the individual studies were not statistically significant, the data, when looked at collectively, points towards a statistically significant difference in the closure rate of the septal occluder and surgical procedure. This tells us that the rate of unsuccessful closure was significantly lower in the surgical group than the septal occluder device group. The heterogeneity in this instance was 69%, indicating a very high level of variability and a low homogeneity in the results of the study. The variability could be due to a number if reasons, as each study was conducted in a different country with different levels of care and experience in surgery, producing different results.
The higher closure rate in the surgical group could be explained through a number of different reasons. One of the main differences is being prepared for the unexpected. With septal occluder devices, prior characteristics must be met in order for the septal occluder to be successful as device closure is not possible if the defect is too large, if multiple defects are present or if it is too near to the blood vessels[67]. When implanting a device, the interventional cardiologist must use imaging techniques in order to safely close the hole, but in certain instances, malpositioning or embolization cause the device to become dislodged. If this occurs, the device must be removed urgently through surgical or catheter techniques[68]. Complications such as these have been known to occur at a rate between 0.01-0.55%, with higher instances when performed by less experienced interventionists[69]. Due to this, when performing this procedure, it is recommended that a surgical team is on stand-by in order to address any postoperative needs if necessary[70]. Taking this into consideration, the likelihood of such treatments like septal occluders may be unviable in developing not purely due to lack of finance; rather it would be the lack of facilities and infrastructure required to make the procedure safe and successful.
6.2 Objective 2
Overall, patients in the septal occluder group were significantly less likely to experience complications than the surgical group as the RD was -0.21, indicating favourable outcomes for the septal occluder. However, as can be seen in figure , around half of the studies analysed did not find any difference in complication rates when comparing the septal occluder group with the surgical group[71]. In this instance, the RD of 0.23 meant that the patients in the septal occluder group were less likely to experience any complications from their procedure than the surgical group. In this group of studies, half crossed the centre line of 1, which is known as the line of no effect[72]. This indicates that the results of those particular studies were not statistically significant to a 95% confidence interval. However, the black diamond, placed at 0.23 [0.19, 0.29], is towards the left of the forest plot and is not touching the line of no effect, thus telling us that the overall evidence points to a significant difference in the rate of complications between the septal occluder and surgical groups.
At 82%, the formal test of heterogeneity, I2, is substantial, signifying a high level of variability in the rate of complications from the studies[73]. Surgeons in developed nations may have more advanced facilities or different levels of experience, as well as a more structured network for postoperative care, leading to less complications[74]. Another factor may be that different studies had different follow-up times. In the study conducted by Butera et al, 2006, a large number of complications were reported in the surgical group (235/533)52. This study included a 2-week follow up where patients were monitored for any postoperative complications, even after discharge. In other studies, the logging period was up until discharge, and thus many minor complications that occurred at home may have gone unrecorded, potentially explaining why the rate of complications is proportionally lower in the majority of the other studies.
The high complication rate in the surgical group is due to a number of factors. The standard surgical intervention of a median sternotomy is far more invasive, making patients susceptible to a number of infections. Out of 317 complications, 16 were infections. It is important to note that this risk is only 0.014%, which when compared to a study in 1984 that estimated the risk as 0.97%, is a significant reduction, highlighting improvements in the operative and postoperative care[75]. The cardiopulmonary bypass machine is also associated with a number of complications[76] however; maintaining a short surgery time minimizes this. Perhaps the most significant reason is the clinical bias associated with surgery where clinicians will recommend a procedure dependent on their need. Patients undergoing surgical closure are more likely to experience more symptoms, have larger defects and more complex pathologies[77].
6.3 Objective 3
6.3.1 Length of hospital stay
All of the five studies that measured length of hospital stay showed a significant difference in the standard mean difference between the two groups as none of the 95% confidence intervals overlapped at the line of no effect at 0. The standard mean difference of the black diamond was -2.56 [-2.69, -2.42]; indicating a significantly shorter stay in hospital for those in the septal occluder group.
This outcome measure can be seen in relation to two things: the rate of complications and costs. An increase in complications has positively correlated with an increased length of hospital stay[78]. The results replicate this as an increase in the rate of complications was seen in the surgical cohort of studies, as was an increased stay in hospital. However, correlation does not necessarily mean causation as a number of other factors could have played a part. For example, in the study conducted by Butera, many patients were kept in hospital for a prolonged period of time; the reason being that many had travelled long distances to reach the hospital11. In Schneeberger’s study, a right anterolateral mini-thoracotomy was used as the cardiac surgery method16. Being a less invasive procedure, one would expect the stay in hospital to be reduced, however, the mean length of stay was 6.10 days, significantly longer than the average of the septal occluder group of 3.00 but shorter than the surgical average of 7.71. In terms of cost, the longer the stay in hospital, the higher the cost. Thus, in addition to procedural costs, the surgical group is more likely to incur additional costs due to their prolonged period of time in hospital.
6.3.2 Cost of procedure
The overall cost was measured in three studies, giving conflicting conclusions. Two of the studies were significantly in favour of surgery whist only one was in favour of the device group, giving an overall value for the standard mean difference as 0.79 [0.47, 1.12]. The black diamond was to the right hand side, indicating lower costs in the surgical group. The three studies all had a similar weighting whilst the heterogeneity was 99%, indicating a high level of variability in the analysis.
Considering each of these occurred in different countries, it is difficult to truly gauge all of the costs, particularly when the data is in USD. For example the study conducted in Guatemala, a low-income country, it was found that surgical closure cost 31% less than device closure18. This dear cost for the occluder group was attributed to the device itself, making up over 65% of the cost per cure at $2930. This is due to high price set by pharmaceutical companies for these occlusion devices, making it less accessible in low-income countries. The study conducted by Kim showed very different results, indicating that the surgical group was more expensive even when the cost of complications was removed13.
In terms of both hospital stay and cost of the procedure, it is clear that the device closure produces far more effective results, particularly with a reduced stay in hospital and less complications in general. This health and financial benefit is clear, but in low-income countries, such procedures with occluder devices would almost be impossible due to the high price tag. Whether this would be financially viable in developing nations is a question that would need answering after further analysis involving more studies and more raw data.
6.4 Limitations
A limitation of my study was the lack of a clear search strategy for each individual objective. By using broad terms as search terms, there was a lack of clear direction when searching for each individual outcome measure. This meant that not all of the relevant studies could have been used, restricting the data that was available for this review.
Another limitation of my study was the inability to differentiate between major and minor complications within the data. Complications were compiled together in many instances and did not specify the number of patients that suffered; rather they assessed the number of complications, some of which may have occurred more than once. If a single patient had suffered multiple complications, it would dramatically skew the results, especially in the smaller studies.
Due to the nature of ASD, an intervention cannot be randomly assigned to patients and thus, all the studies were retrospective cohort studies. Patients were assigned to either the septal occluder group or surgical group within a specified period of time looking at certain outcome measures. This review has collated the data collected from these studies to provide evidence as to which of the two procedures is safer, more efficacious and financially viable.
One of the glaring compromises of all the studies analysed is that the data is non-randomised. Patients were assigned to a group depending on their clinical need, and preference was often taken into account leaving room for bias amongst patients and practitioners alike. Within the studies that were analysed, there were several instances where women opted for less invasive procedures in order to reduce long term scarring and improve aesthetic appearance[79]. A geographical bias may also be seen where patients who cannot access a tertiary care centre are not treated using an occluder device
Another issue is that these studies occurred over a period of years at a time and thus modes and methods of treatments may change, influencing the results. In the vast majority of cases the Amplatzer Septal Occluder was used or a median sternotomy was performed, thus minimizing the influence of other factors within the procedure itself. Other developments such as newer drugs or more advanced equipment could have decreased the risk of complications or reduced the time spent in hospital, causing discrepencies within the sample population. Human error may also play a significant part in influencing the results, for example, decisions made by doctors who are not used to working in cardiology could play a part in causing an increase in the rate of complications and subsequently the length of stay in hospital.
The size of the studies varies dramatically with the largest involving 1286 participants and the smallest involving only 53. Smaller studies are more at risk of being affected by outliers in their results and are more likely to skew the overall trend of the results, making it more difficult to make conclusions from the data. With larger sample sizes, the data is considerably more consistent and precise, particularly when the effect size is small, as a small effect size would only be significant in a large sample size[80].
7. Recommendation and Conclusion
One of the ways to make the data more comparable would be to group patients in terms of their clinical need and characteristics, randomly assigning each patient to a different group if possible. This would allow bias to be removed to an extent and allow the analysis of how effective each treatment is in different groups. Interestingly, each of my studies took place in a different country, leading to variability in the results. It would be far more reliable to have studies taking place in similarly equipped hospitals with similar post-operative procedures in order to make each study as similar as possible to the last.
Therefore to conclude, there is strong evidence to suggest that both these procedures are safe and effective for patients. Septal occluders have proven to be effective in reducing the rate of complications and length of hospital stay drastically, however it still does not compare to the high closure rate seen in the surgical group. The cost for each procedure was inconclusive as studies occurred in different health economies where some procedures are more readily available than others.
This review has also highlighted the need for a less invasive approach for the treatment of ASD, whilst still giving surgeons full access to the heart. Although minimally invasive cardiac surgery is a relatively new concept in treating ASD, it has the potential to maintain a high success rate in closure whilst reducing the post-operative complication due to it’s less invasive approach.
8.References
[1] Webb G, Gatzoulis MA. Atrial septal defects in the adult. Circulation 2006; 114: 1645-1653.
[2] Khan AA, Tan JL, Li W, Dimopoulos K, Spence MS, Chow P et al. The impact of transcatheter atrial septal defect closure in the older population: a prospective study. J Am Coll Cardiol 2010; 3: 276–281.
[3] Linde DV, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Johanna JJM et al. Birth prevalence of congenital heart disease worldwide. J Am Coll Cardiol. 2011; 58: 2241-47.
[4] Webster MW, Smith HJ, Sharpe DN, Chancellor AM, Swift DL, Bass NM, Glasgow GL. Patent foramen ovale in young stroke patients. The Lancet. 1988 Jul 2;332(8601):11-2.
[5] Oliver JM, Gallego P, Gonzalez A, Dominguez FJ, Aroca A, Mesa JM. Sinus venosus syndrome: atrial septal defect or anomalous venous connection? A multiplane transoesophageal approach. Heart. 2002; 88: 634–638.
[6] Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R. Sinus venosus defects: unroofing of the right pulmonary veins: anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994; 128: 365–379.
[7] Bertram H, Paul T, Kaulitz R, Luhmer I, Kallfelz HC. Coronary sinus defects: rare form of interatrial communication. Zeitschrift fur Kardiologie. 1996 Dec;85(12):899-905.
[8] Kumar KJ, Clark ML. Kumar & Clark’s clinical medicine, 9th ed. London: Elsevier; 2017.
[9] Brickner ME, Hillis LD, Lange RA, Congenital heart disease in adults. First of two parts. N Engl J Med. 2000; 342: 256-63.
[10] Siddiqui WT, Usman T, Atiq M, Amanullah MM. Transcatheter versus surgical closure of atrial septum defect: A Debate from a Developing Country. J Thorac Cardiov Sur. 2014; 6: 205-10.
[11] King TD, Thompson SL, Steiner C, et al. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976; 235 :2506–9.
[12] Rosas M, Attie F . Atrial septal defect in adults. Timely Top Med Cardiovasc Dis. 2006; 11: 34.
[13] H. Rostad, S.J. Sørland. Atrial septal defects of secundum type in patients less than 40 years of age: a follow-up study. Acta Medica Scand. 1981; 645: 29–35.
[14] Figure 1: http://www.heart-valve-surgery.com/heart-surgery-blog/2008/02/12/open-heart-surgery-diagram-after-chest-incision-and-sternotomy/ [Date accessed: 03/04/17]
[15]Figure 2: http://accessemergencymedicine.mhmedical.com/content.aspx? bookid=683§ionid=45343680 [Date accessed: 03/04/17]
[16] Figure 3: https://medmovie.com/topic/cvml_0460a/2999_09_amplatzer_1920x1242/ [Date accessed: 03/04/17]
[17] Sackett DL, Rosenberg WM, Gray JA. Evidence based medicine: what it is and what it isn’t. BMJ. 1996; 312: 71-72.
[18] Figure 4: https://drkokogyi.wordpress.com/2013/11/07/many-ladies-used-to-misunderstand-the-implantation-bleeding-with-their-periods-and-miscalculated-the-length-of-their-pregnancy/ [Date accessed: 05/04/17]
[19] Figure 5: https://lithopedionbaby.wordpress.com/physiology/implantation-of-the-egg/ [Date accessed: 06/04/17]
[20] Figure 6: http://www.rci.rutgers.edu/~uzwiak/AnatPhys/APSpringLect20.html [Date accessed: 08/04/17]
[21] Le T, Vikas Bhushan V, Sochat M, Chavda Y. First Aid for the USMLE Step 1, 27 ed. London: McGraw-Hill Education – Europe; 2017
[22] Figure 7: https://dundeemedstudentnotes.wordpress.com/2012/05/01/early-embryology/ [Date accessed: 08/04/17]
[23] Figure 8: http://slideplayer.com/slide/5282023/ [Date accessed: 10/04/17]
[24] Figure 9: http://www.vcbio.science.ru.nl/en/virtuallessons/embryology/chicken-33-36h/ [Date accessed: 12/04/17]
[25] Moorman A, Webb S, Brown NA, Lamers W, Anderson RH. Development of the heart:(1) formation of the cardiac chambers and arterial trunks. Heart. 2003; 89: 806-14.
[26] Figure 10: http://cnx.org/content/col11496/1.6/ [Date accessed: 12/04/17]
[27] Naish J, Revest P, Syndercombe Court D. Medical Sciences. Edinburgh: Saunders Elsevier; 2009.
[28] Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circulation research. 2006; 95: 261-8.
[29] Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nature Rev Genet. 2005; 6 : 826-37.
[30] Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009; 336: 137-44.
[31] Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004; 18: 99-115.
[32] Calkoen EE, Hazekamp MG, Blom NA, Elders BB, Gittenberger-de Groot AC, Haak MC, Bartelings MM, et al. Atrioventricular septal defect: From embryonic development to long-term follow-up. Int J Cardiol. 2016; 202: 784-95
[33] Kramer TC. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. Am J Anat. 1942; 71: 343-70.
[34] Figure 11: https://www.studyblue.com/notes/note/n/embryology-cardio/deck/12137513 [Date accessed: 12/04/17]
[35] Anderson RH, Webb S, Brown NA, Lamers W, Moorman A. Development of the heart:(2) Septation of the atriums and ventricles. Heart. 2003; 89: 949-58.
[36] Wang Y. Vascular biology of the placenta. InColloquium Series on Integrated Systems Physiology: from Molecule to Function. 2010 (Vol. 2, No. 1, pp. 1-98). Morgan & Claypool Life Sciences.
[37] Figure 12: http://schoolbag.info/biology/mcat/15.html [Date accessed: 12/04/17]
[38] Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998; 281: 108-11.
[39] Perrino C, Rockman HA. GATA4 and the two sides of gene expression reprogramming. Circulation Res. 2006; 98: 715-716.
[40] Sarkozy A, Conti E, Neri C, d’Agostino R, Digilio MC, Esposito G, et al. Spectrum of atrial septal defects associated with mutations of NKX2. 5 and GATA4 transcription factors. J Med genet. 2005; 42: e16-19.
[41] Celermajer DS, Bull C, Till JA, Cullen S, Vassillikos VP, Sullivan ID, Allan L, Nihoyannopoulos P, Somerville J, Deanfield JE. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994; 23: 170-6.
[42] Smith AT, Sack GH, Taylor GJ. Holt-oram syndrome. J Pediatr. 1979; 95: 538-43.
[43] Lang F, editor. Encyclopedia of molecular mechanisms of disease. Springer Science & Business Media; 2009.
[44] Sambhi MP, Zimmerman HA. Pathologic physiology of Lutembacher syndrome. Am J Cardiol. 1958; 2: 681-6.
[45] Allanson JE. Noonan syndrome. J Med Genet. 1987; 24: 9.
[46] Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J med genet. 1998: 80: 213-7.
[47] Burd L, Deal E, Rios R, Adickes E, Wynne J, Klug MG. Congenital Heart Defects and Fetal Alcohol Spectrum Disorders. Congenit Heart Dis. 2007; 2: 250–255.
[48] Tortora GJ, Derrickson B. Principles of Anatomy & Physiology. 14th, EMEA ed. Hoboken, NJ: Wiley; 2014.
[49] Vongpatanasin W, Brickner ME, Hillis LD, Lange RA. The Eisenmenger syndrome in adults. Ann Int Med. 1998; 128: 745-55.
[50] Waddell TK, Bennett L, Kennedy R, Todd TR, Keshavjee SH. Heart–lung or lung transplantation for Eisenmenger syndrome. J Heart Lung Transplant. 2002; 21: 731-7.
[51] Loscalzo J. Paradoxical embolism: clinical presentation, diagnostic strategies, and therapeutic options. Am Heart J. 1986; 112: 141-5.
[52] Rakhit RD. Case 2: patent foramen ovale (PFO) and paradoxical embolism. Heart. 2003; 89: 1362.
[53] Barber JM, Magidson O, Wood P. ATRIAL SEPTAL DEFECT: With special reference to the Electrocardiogram, the Pulmonary Artery Pressure and the Second Heart Sound. Brit Heart J. 1950; 12: 277.
[54] Griffiths CJ, Murray A, Hunter S. Computer assisted M-mode echocardiogram analysis. Clin Phys Physiol M. 1982; 3: 103.
[55] Pryor R, Woodwark GM, Blount SG. Electrocardiographic changes in atrial septal defects: ostium secundum defect versus ostium primum (endocardial cushion) defect. Am Heart J. 1959; 58: 689-700.
[56] Figure 13: http://www.healio.com/cardiology/learn-the-heart/ecg-review/ecg-topic-reviews-and-criteria/atrial-septal-defect-review [Date accessed: 03/04/17]
[57] Longmore, J. M. (J. Murray), Wilkinson I, Turmezei T, Cheung CK. Oxford Handbook of Clinical Medicine. 7th ed. Oxford: Oxford University Press; 2007.
[58] Bettencourt N, Salomé N, Carneiro F, Gonçalves M, Ribeiro J, Braga JP. Atrial septal closure in adults: surgery versus amplatzer–comparison of results. Rev Port Cardiol 2003; 22: 1203-11.
[59] Butera G, Carminati M, Chessa M, Youssef R, Drago M, Giamberti A, et al. Percutaneous versus surgical closure of secundum atrial septal defect: comparison of early results and complications. Am Heart J. 2006; 151: 228-34.
[60] Hoashi T, Yazaki S, Kagisaki K, Kitano M, Kubota SM, Shiraishi I, et al. Management of ostium secundum atrial septal defect in the era of percutaneous trans-catheter device closure: 7-year experience at a single institution. J Cardiol. 2015; 65: 418-22.
[61] Kim JJ, Hijazi ZM. Clinical outcomes and costs of Amplatzer transcatheter closure as compared with surgical closure of ostium secundum atrial septal defects. Med Sci Monit. 2002; 8: 787-91.
[62] O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J. 2015; 169: 727-35.
[63] Ruzicka P, Malik P, Cerny J, Kucera D, Homza M, Kala P, et al. Comparison of catheterisation and surgical treatment of ostium secundum type atrial septal defect in adult patients. Vnitrni Lekarstvi. 2005; 51: 1079.
[64] Schneeberger Y, Schaefer A, Conradi L, Brickwedel J, Reichenspurner H, Kozlik-Feldmann R, Detter C. Minimally invasive endoscopic surgery versus catheter-based device occlusion for atrial septal defects in adults: reconsideration of the standard of care. Interact Cardiovasc Thorac Surg. 2016; 1: ivw366.
[65] Suchon E, Pieculewicz M, Tracz W, Przewlocki T, Sadowski J, Podolec P. Transcatheter closure as an alternative and equivalent method to the surgical treatment of atrial septal defect in adults: comparison of early and late results. Med Sci Monit. 2009; 15: 612-7.
[66] Vida VL, Barnoya J, O’Connell M, Leon-Wyss J, Larrazabal LA, Castañeda AR. Surgical versus percutaneous occlusion of ostium secundum atrial septal defects: results and cost-effective considerations in a low-income country. J Am Coll Cardiol. 2006; 47: 326-31.
[67] Berger F, Vogel M, Alexi-Meskishvili V, Lange PE. Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. J Thorac Cardiovasc Surg. 1999; 118: 674-80.
[68] Spence MS, Qureshi SA. Complications of transcatheter closure of atrial septal defects. Heart. 2005; 91: 1512-1514
[69] Son J-W, Park J-S. Subacute, Silent Embolization of Amplatzer Atrial Septal Defect Closure Device to the Pulmonary Artery. J Cardiovasc Ultrasound. 2012;20(4):201-204.
[70] Amanullah MM, Siddiqui MT, Khan MZ, Atiq MA. Surgical rescue of embolized amplatzer devices. J Card Surg. 2011; 26: 254-8.
[71] Ried K. Interpreting and understanding meta-analysis graphs–a practical guide.Aust Fam Physician.2006; 35: 635-8.
[72] Verhagen AP, Ferreira ML. Forest plots. J. Physiother. 2014; 60: 170–173.
[73] Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21: 1539-58.
[74] Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. Changes in safety attitude and relationship to decreased postoperative morbidity and mortality following implementation of a checklist-based surgical safety intervention. BMJ quality & safety. 2011; 20:102-7.
[75] Grossi EA, Culliford AT, Krieger KH, Kloth D, Press R, Baumann FG, et al. A Survey of 77 Major Infectious Complications of Median Sternotomy: A Review of 7,949 Consecutive Operative Procedures. Ann Cardiothorac Surg. 1985; 40: 214-223
[76]Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983; 86: 845-57.
[77] Galal MO, Wobst A, Halees Z, Hatle L, Schmaltz AA, Khougeer F, De Vol E. Peri-operative complications following surgical closure of atrial septal defect type II in 232 patients—a baseline study. Eur Heart J. 1994; 15: 1381-4.
[78] Krell RW, Girotti ME, Dimick JB. Extended length of stay after surgery: complications, inefficient practice, or sick patients? JAMA surg. 2014; 149: 815-20.
[79] Yoshimura N, Yamaguchi M, Oshima Y, Oka S, Ootaki Y, Yoshida M. Repair of atrial septal defect through a right posterolateral thoracotomy: a cosmetic approach for female patients. Ann Cardiothorac Surg. 2001; 72: 2103-5.
[80] Anzures-cabrera J, Higgins JP. Graphical displays for meta-analysis: An overview with suggestions for practice. Res Synth Methods. 2010; 1: 66-80.
#dissertation #thesis #essay #assignment #phd #phdstudent #phdlife #essaywriting #research #phdjourney #dissertationlife #gradschool #assignments #phdproblems #academicwriting #assignmenthelp #thesiswriting #gradstudent #dissertationproblems #university #academiclife #gradschoolproblems #academia #researchpaper #gradschoollife #graduateschool #college #doctoralstudent #dissertationdone #writing





