The estimated reading time for this post is 19 Minutes
Abstract
Cilia are specialized structures with distinctive function. Cilia maintain a specific protein array performing precise functions. The accuracy of traffic of proteins to and from cilia ensure proper functioning of the involved signaling cascades. It is not completely understood how the proteins traffic to cilia. In this review, we will focus how lipid modifications on proteins plays a role in cilia protein trafficking and regulation of signaling pathways. About 30% of cilia proteins are acylated. Lipid modifications likely have a role more than just tethering the proteins to membrane. Lipid modifications on proteins might help them travel to cilia by facilitating interaction with transporter proteins. Lipid modifications can also regulate protein stability and abundance. Future studies focusing on role of lipid modifications in cilia biology will help understand how cilia maintain a complement of specific proteins that allows for specific functions.
Keywords: Palmitoylation, Myristoylation, Prenylation, Acylation, Ciliopathies
Background
The primary cilium is an organelle that protrudes from the surface of the cell and functions as a signaling center. They are non-motile, solitary, polarized and mainly serve as a sensory organelle for the cell. It is found on almost every cell type in vertebrates and has been linked to a variety of human diseases, collectively referred to as ciliopathies.
The output of the genetic blueprint of human body is far more complex and varied then the genes themselves. Nature is a perfect conductor who has over two million proteins of human body to function in an orchestrated fashion so as to maintain absolute homeostasis. The function of proteins is modulated and diversified by post-translational modifications (PTMs) such as phosphorylation, glycosylation, ubiquitination, nitrosylation, methylation, acetylation, lipidation and proteolysis (1). These covalent alterations of amino-acid side chains have role in all the aspects of cell biology.Thus, understanding PTMs is important for understanding and prevention of human diseases.
Cilia maintain specific protein array by selective trafficking of proteins to and from cilia. How proteins travel to cilia is still being explored. In this review, we will focus how lipid modifications are involved in specific cilia protein trafficking and regulation of signaling pathways. Lipid modifications on proteins might help them travel to cilia by two (possibly overlapping) mechanisms:
(a) facilitate binding of cilia targeted proteins to membranes to allow for delivery to cilia or
(b) facilitation of interactions with specific carrier chaperons which deliver the protein to cilia. The accuracy of traffic of proteins to and from cilia will ensure proper functioning of the involved signaling cascades.
For structural integrity and function cilia requires a specific array of protein. Primary cilium is reported to have a high density of receptors that play an important role in perceiving extracellular stimuli. Though continuous with cytosol, cilia provides a separate cellular compartment for unique spatial and temporal regulation of signaling molecules. Primary cilia are critical for a number of signaling pathways: Wnt signaling, calcium signaling, growth factor signaling, Shh signaling, G-protein coupled receptor signaling and receptor tyrosine kinase signaling ((2) https://ciliajournal.biomedcentral.com/articles/10.1186/2046-2530-1-4). Accurate localization of signaling proteins confers proper functioning of the pathways.
As no study till date have shown that proteins are produced in cilia, the specific array of protein is maintained by regulated protein traffic to cilia. The trafficking of proteins to cilia is complex and many mechanisms have been put forward to explain the phenomenon. There exists a peri-ciliary diffusion barrier that separates the ciliary and plasma membrane despite the continuity between the two (3). Along with lateral transport, specific traffic at the base of the cilium is believed to occur. At the base of cilia there is a transition zone which might regulate the entry of proteins into cilia. It has been proposed that like the nuclear pore, a ciliary pore exists in this transition zone that controls entry of proteins into the cilia (4). This hypothesis is consistent with the findings that ciliary entry of a cytoplasmic protein, the kinesin-2 motor KIF17 and X-linked retinitis pigmentosa protein (RP2) requires an import signal similar to a nuclear localization signal (NLS) (5, 6). Small GTPase, Ran and its binding partners, the importins, have been implicated in regulating cilia entry of specific proteins (7, 8). Bardet Biedl syndrome (BBS) proteins form a complex called BBSome which particularly localize to the basal body of cilia and centrosomes. It has been proposed that the BBSome form a protein coat on vesicles helping transport of proteins to the cilia (9).
Protein trafficking is also reported to be facilitated by presence of specific cilia targeting sequence (CTS) however there is no unique CTS, suggesting more than one molecular mechanism is involved. The best characterized CTS is RVxP which exists on Polycystin 2 and Rhodopsin (7). Ax[S/A]xQ motif has been reported to occur on several G protein–coupled receptors (10). Apart from CTS, lipid modifications have been identified as a requirement for trafficking of a number of ciliary proteins a few of which will be discussed here. About 30% of cilia protein are likely to be acylated (11). With more protein acylation studies being done, it is becoming clearer that acylation of cilia protein is more than mere membrane attachment. Along with membrane tethering they are also integral for proper localization, distribution, abundance, stability and function of several cilia protein (11-16). In the following sections role of various lipid modifications (table 1) on cilia protein trafficking and signaling will be discussed.
Palmitoylation
The role of one of the PTMs that has huge impact on cellular functions but is less studied is palmitoylation. Palmitoylation is a lipidation process where a C16 palmitoyl group from palmitoyl-CoA gets added to the thiolate side chain of cysteine residues via palmitoyl acyl transferases (PATs) (17). Palmitoylation is unique because of its reversible nature and the great number of transferases and thioesterases involved with the process. This modification can anchor proteins to the membrane and thioesterases can break the link between the protein and the lipid endowing it as an on/off switch to regulate membrane localization (18, 19). The reversibility of palmitoylation makes it a candidate regulator for localization and function of many proteins.
Several proteins require attachment of a palmitoyl group for proper localization which in turn effects its abundance and function. Ciliary proteins are no different. The CTS of fibrocystin and the lipid raft targeting sequence of SNARE 25 has three and four palmitoylated cysteine residues respectively (20, 21). The targeting sequences alone without palmitoylation are not sufficient for ciliary targeting making it reasonable to believe that palmitoylation might help in restricting the proteins to specific lipid domains and help in recruitment of sorting complexes for delivery to cilia.
Arl13b is a GTPase localized primarily in cilia and necessary for proper cilia formation. Arl13b is palmitoylated at its N terminus which is essential for its localization to cilia, stability along with its function in cilia elongation and formation (11). Though Palmitoylation is absolutely necessary for its cilia elongation function, it localization to cilia needs the C terminus also (11).
Rhodopsin is a seven transmembrane protein and is the primary photoreceptor molecule of vision (22, 23). It is synthesized and folded in endoplasmic reticulum and is trafficked to the membrane. Role of the palmitoylation of rhodopsin remain unclear but it is thought to orient the protein near the membrane in such a manner to provide maximum interaction with transport components, facilitating its trafficking (24). Palmitoylation has been reported to play a role in the stability of opsin: an unliganded form of rhodopsin. Lack of opsin palmitoylation causes its mislocalization and rapid rod photoreceptor degeneration (23).
PKD1 and PKD2 encode the proteins PC1 and PC2. When PKD1 and PKD2 are mutated they cause autosomal dominant polycystic kidney disease (ADPKD) which is characterized by numerous renal cysts which progressively leads to loss of renal function. Recently we reported that polycystin1 (PC1) and not polycystin2 (PC2), which are both polytopic integral membrane proteins and localize to primary cilia, is palmitoylated (25). Data indicate that palmitoylation is important for its localization and abundance.
A very recent study by Xu Wu group from Harvard Medical School has shown that the transcription factor, Regulatory Factor X 3 (RFX3) is S-fatty acylated on a cysteine residue which is evolutionarily conserved in the dimerization domain. RFX3 is one of the key transcription factors involved in cilia formation and function. The acylation regulates protein interaction network by affecting homo- or hetero- dimerization, thereby regulating ciliogenesis as well as tissue specification. Deregulation of fatty acid metabolism might affect fatty acylation of RFX3 and thereby the RFX3-mediated protein interaction network, leading to ciliopathies and metabolic disorders such as diabetes (26).
Hedgehog signaling is an evolutionary conserved pathway having important role in numerous organ development and cancer (27). Lipid modifications on hedgehog (Hh) protein regulates its function and distribution. It has palmitate in an amide linkage with N terminal cysteine and there is a cholesterol moiety addition on the C terminus. The acylation efficiency and specificity depends in part upon prior cholesterol modification (28). The duel modifications provide higher membrane affinity of Hh thus allowing its association to sterol-rich membrane microdomains in Drosophila, and to lipid rafts in mammalian cells. The association provides a platform for intracellular sorting, signal transduction and restricting the range of its activity. However just membrane affinity must not be the only function of the lipidation process as removal of N terminal cysteine and hence acylation results in large reduction in signaling function. The N terminus might be needed for modulating additional interactions needed for the complete signaling process. R. Blake Pepinsky’s group had proposed a possibility that the N-terminal region might be important for modulating the Ptc-1/Smo interaction and that it’s truncated forms, although can bind to Ptc-1, are unable to trigger the de-repression of Smo (29, 30).
Another important signaling pathway associated with cilia is Wnt pathway. Wnt proteins are palmitoylated giving the protein its hydrophobic character and is important for its extracellular transport. Murine Wnt-3a is also modified with a monounsaturated fatty acid, palmitoleic acid at a conserved serine residue (Ser209) (31). The enzymatic removal of palmitate or cysteine mutation leading to loss of palmitate, reduced the biological activity of the protein indicating the lipid modification is integral part for the signaling pathway (30, 32).
Myristoylation
Myristoylation is the covalent attachment of a 14-carbon saturated fatty acid, myristate, to the N-terminal glycine of proteins by N-myristoyltransferase. It can occur on a variety of proteins involved in diverse function and localized variedly. It is an irreversible protein modification that generally occurs co-translationally following removal of the initiator methionine residue by methionylaminopeptidases. Myristoylation can also occur post-translationally, as in the case of many apoptotic proteins where proteolytic cleavage by caspases reveals an internal myristoylation motif (15).
Myristoylation has been reported to help a number of cilia protein with membrane association required for cilia localization, however it is not sufficient alone. Presence of a second binding partner or a chaperon protein in the process is required.
Myristoylation along with the presence of specific CTS has been implicated in traffic of many cilia proteins. Cystin, is a 145–amino acid (aa) cilium-associated protein responsible for autosomal recessive Polycystic kidney disease (PKD). Like other cystoproteins (eg polycystin 1) its expression is developmentally regulated. Cystin is myristoylated at its G2 residue and helps in its membrane association. It has been shown that this acylation is required for its proper localization to the ciliary membrane (33). However, acylation is not always sufficient for cilia targeting. Cystin has a unique AxEGG motif necessary to target and retain in cilium.
Cilia localization of another protein Nephronophthisis 3 (NPHP 3) requires a coiled coil (CC) domain and myristoylation at the N terminus. Nephronophthisis is an autosomal recessive kidney disease with 11 causative genes identified till date (NPHP 1-11). Mutations in NPHP 3 cause human NPHP type 3. The mouse Nphp3 gene product is a 140 kDa protein (1325 amino acids) with multiple domains, including three coiled-coil (CC) domains and eight tetratricopeptide repeats (TPRs). The CC domain is required to transport NPHP 3 to the basal body and myristoylation is required for its subsequent transport into the ciliary shaft. Secondary binding partners might interact with the CC or the myristoylated region to facilitate the process but that mechanism is still unknown (34). NPHP 3 also has been implicated in both canonical and noncanonical Wnt signaling (35, 36). Hence proper lipid modification on it and its accurate locatization to cilia is important for ciliary signaling.
An interesting mode of chaperon mediated myristoylated and prenylated protein delivery system to cilia have been identified recently. Prenylated protein details is discussed in later section. Two novel proteins UNC119A and UNC119B have been shown to act as chaperons which have specificity for a diverse subset of myristoylated proteins such as G protein α subunits, NPHP3 and src- type tyrosine kinase, and deliver them to cilia. UNC119 polypeptides bind lauroyl (C12) and myristoyl (C14) side chains via a hydrophobic pocket formed by an immunoglobulin-like β-sandwich fold. Once the cargo bound chaperons reach cilia, Arl3-GTP releases the cargo from the chaperon, delivering the proteins to the cilia (37-39). NPHP3 targeting to cilia requires ARL3, UNC119B (not UNC119A) and the ARL3 GAP RP2. Knockdown of the Arl3 system reduced the percentage of cilia with localized NPHP3 (40). This is a sophisticated delivery system where the lipids do not merely act as a membrane tethering chain but a specific binding site for protein-protein interactions.
Cilium both releases and binds extracellular vesicles (EV). Myristoylation has been linked to signal target proteins to EVs in Jurkat T cells (41). CIL-7 a ciliary protein, regulates EV biogenesis and is required for polycystin mediated sensory signaling. Recent studies in C. elegans have also indicated that myristoylation is important for CIL-7 function and its targeting to EVs (42).
Double acylation: Myristoylation and Palmitoylation
Non-receptor tyrosine kinases, G protein subunits and G protein regulators (43), (16) show more than one lipid modifications which modulate their localization and function. More than one acylation is shown by cilia proteins too for correct membrane and subcellular localization including cilia. Myristoylation and palmitoylation for membrane anchoring and for ciliary localization of proteins is seen in eukaryoticflagella, C. elegans sensory neurons, mammalian photoreceptors and retinal pigment epithelial cells (24, 44, 45)
Double acylation helps in strong membrane association. Unlike popular belief, strong membrane association, in many cases it is not sufficient for cilia localization. Presence of double acylation on various proteins may confirm specific binding partners or localization for specific functions. CePPEF, the only PPEF family of serine/threonine protein phosphatases encoded within the C. elegans shows adjacent N-terminal myristoylation and palmitoylation for proper cilia trafficking. As palmitoylation is sufficient for membrane binding the functional consequence of having both modifications are unclear. Ramulu and Nathans provide an explanation that in C. elegans, palmitoylation of CePPEF may be heterogeneous such that one subset of CePPEF proteins is palmitoylated and strongly membrane-associated, and a second subset of CePPEF proteins carries only a myristate group and can therefore shuttle between the membrane and cytosol (44).
A flagellar protein Calflagin or its homolog FCaBP (Flagellar calcium-binding protein) is myristoylated and palmitoylated and these modifications are necessary for its flagella localization. It is a unique protein that uses calcium-acyl switch for regulated membrane attachment and probable association with interactors like protein recoverin. Recoverin is myristoylated in the calcium-bound state and associates with the plasma membrane via interactors, when calcium level drops myristoyl group becomes sequestered in a hydrophobic cleft leading to loss of membrane association. The calcium-binding state of recoverin regulate the membrane accessibility of its fatty acid, which in turn modulate the binding of recoverin to its partner proteins (12, 46-48). The localization of FCaBP is predicted to result in part from an association with the plasma membrane through its myristoyl group and also from an association with a partner protein through palmitate (46). For RP2 protein also lipidation does more than just membrane association. RP2 is mutated in X-linked retinitis pigmentosa. It localizes to ciliary base and associates with membrane by dual acylation of the N-terminus: myristoylation at Gly2 and palmitoylation at Cys3. RP2 traffics to cilia by interacting with Importin protein and membrane association of RP2 is a prerequisite for formation of an RP2–Importin-β2 complex (6).
Recent study from Yoshimura’s lab has shown that similar to molecular targeting signal for motile flagella, a combination of myristoylation and palmitoylation targets proteins such as RP2, Lck, Fyn and Yes-1 to the primary cilium in eukaryotic cells. The targeting is mediated by an N-terminal dual lipidation-coupled ciliary targeting signal (nlCTS). Palmitoylation of nlCTSs appears to be important not only for ciliary targeting but also for the stability of the respective proteins (13).
Prenylation: Farnesylation and Geranylgeranylation
Prenylation is a lipid modification where a farnesyl (15-carbon) or geranylgeranyl (20-carbon) isoprenoids is covalently added to conserved cysteine residues at or near the C-terminus of proteins. Apart from facilitating membrane attachment for prenylated proteins it is also known to be involved in protein-protein interactions influencing protein function and localization; example Ras family member proteins (p21Kras4B and p21rhoA). In fact, it is believed that lipid association is just a part of the process by which prenylated proteins associate with membranes. They generally interact with receptor molecules in the membranes (14, 49). As more studies are coming up supporting the theory, it indicates that prenylated molecules associate with specific membranes via receptor molecules apart from lipid attachment and interaction with these receptor molecules in few cases are more important for the protein function and localization than just membrane tethering.
A few cilia proteins are known to be prenylated. Proper localization of those proteins and their functions are attributed to this lipid attachment. Inherited retinal diseases has been associated with mutations causing prenylation defects in proteins such as AIPL1, PDE6D and rab escort protein-1 (REP-1) . (50). Inositol polyphosphate-5-phosphatase B (INPP5B) is an important ciliary protein knockdown of which leads to cilia defects in zebrafish. For proper cilia localization it needs to be prenylated at its C-terminus (51). Deletion of CAAX motif leads to abolition of INPP5B recruitment to cilia and also reduces cilia length indicating the CAAX domain is important for INPP5B in the formation of primary cilia. In fact, the CAAX motif was found to play a role in MORM syndrome, a type of ciliopathy, where a mutation caused premature truncation of the protein causing the protein to lose the C terminal CAAX domain (52).
Studies from last few years has provided interesting evidence that prenylated proteins are trafficked to cilia with the help of chaperon protein PDEδ similar to myristoylated proteins (53, 54). PDEδ is known to be involved in the outer-segment targeting via the connecting cilium, of PDE6 α/β subunits, transducin γ subunit, and GRK1 in photoreceptors. The proteins are either partly mis-localized or are degraded in PDEδ −/− photoreceptors (53, 55). However, the understanding of the mechanistic aspect of the role of PDEδ in trafficking of proteins was limited. Recently Wittinghofer and group showed that the lipid moiety of farnesylated Rheb is deeply buried in a hydrophobic pocket of PDEδ indicating binding occurs through prenyl group in the C terminus (53). Retinitis pigmentosa GTPase regulator (RPGR) an interacting partner for PDEδ protein. It is a prenylated protein and acts as GTP-GDP exchange factor for Rab8, a protein important for ciliary protein transport. Geranylation is necessary for the correct localization of RPGR in the Golgi apparatus and cilia. RPGR is prenylated at the C terminus and mutation of the CaaX prenylation motif abrogate the localization.
Structural studies suggest that RPGR helps to recruit prenylated ciliary cargo proteins on PDEδ at the ciliary transition zone. The cargo is delivered to cilia where they are released from the chaperon by Arl2 (and Arl3) by induction of low affinity rapidly dissociating ternary complex similar to myristoylated cargos mentioned earlier (53, 56). Arl13b acts as a GEF for Arl3 and activates Arl3 in cilia which release the cargo from the carrier protein (57). Inositol polyphosphate-5-phosphatase E (INNP5E) is also a ciliary protein and is involved in the Joubert’s syndrome. Unlike INNP5B it has a CTS (FDRELYL) along with a CAAX domain and the CTS is sufficient for its ciliary localization (58). INNP5E associates with phosphodiesterase 6δ (PDE6δ) to traffic to basal body but do not require Arl3 or Arl2 but Arl13b for its unloading from PDE6δ and cilia entry.
INPP5B interacts with both Arl13b and PDE6δ individually but Arl13b and PDE6δ never interacted physically. This indicated Arl13b facilitated ciliary targeting of INPP5E by directly interacting with INPP5E and promoting its release from PDE6δ, unlike ARL3–PDE6δ interaction (58). The structural studies further strengthen the role of lipid as more than just membrane binding moiety. Though new models are being proposed (58, 59), detailed mechanism for cilia trafficking of several prenylated proteins like PDE6 α/β and GNGT1 remain to be determined.
Conclusion and future questions
Cilia being a specialized structure maintains a specific array of molecules. Much attention has been paid to understand how the protein molecules get sorted to cilia to carry out its precise role. More than one transport mechanism are involved in the process. Targeting of proteins to cilia generally relies on partitioning into specific lipid microdomains and on the recruitment of specific sorting complexes. A great number of studies have shown that lipid modifications on the proteins has an important role to play in trafficking as well as in the cilia biology including signaling pathways. Lipid modifications might have a role far more than just tethering the proteins to the membrane. Though a number of cilia proteins are acylated, the complete understanding of the role of lipids in the plethora of protein transport, function and localization in cilia is still a mystery. Future detailed studies on protein modifications and their role in cilia will elucidate some of the unknown.
List of abbreviations
ADPKD – Autosomal dominant polycystic kidney disease
ARL3 – ADP Ribosylation Factor like GTPase 3
BBS – Bardet Biedl syndrome
CC – Coiled coil
CIL-7 a ciliary protein
CTS – Cilia targeting sequene
EV – Extracellular vesicles
FCaBP – Flagellar calcium-binding protein
GAP – GTPase activating protein
GNGT1 – Guanine nucleotide-binding protein G(T) subunit gamma-T1
GRK1 – G Protein-Coupled Receptor Kinase 1
GTP – Guanosine triphosphate
GTPase – Guanosine triphosphatase
Hh – Hedgehog
INNP5E – Inositol polyphosphate-5-phosphatase E
INPP5B – Inositol polyphosphate-5-phosphatase B
KIF17 – Kinesin Family Member 17
nlCTS – N-terminal dual lipidation-coupled ciliary targeting signal
NPHP – Nephronophthisis
PATs – palmitoyl acyl transferases
PC1 – Polycystin1
PC2 – Polycystin2
PDE6δ – Phosphodiesterase 6δ
PKD – Polycystic kidney disease
PPEF – Protein phosphatases with EF-hand domains
PTM – Post-translational modifications
REP-1 – Rab escort protein-1
RFX 3 – Regulatory Factor X 3
RP2 – Retinitis pigmentosa protein
RPGR – Retinitis pigmentosa GTPase regulator
SNARE 25 – Synaptosomal-associated protein 25
TPRs – tetratricopeptide repeats
Table 1: Commonlipid modifications at a glance.
| Palmitoylation | Myristoylation | Prenylation | |
|
Structure |
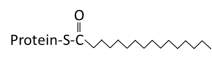
Saturated 16 carbon palmitate addition to cysteines on the targeted proteins via the formation of a thioester linkage |
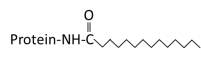
Saturated 14 carbon myristoylated group of an N terminal glycine |
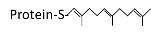
Transfer of either a farnesyl or a geranyl- geranyl moiety to C terminal cysteine(s) of the target protein |
|
Reversibility |
Reversible | Irreversible | Irreversible |
|
Enzymes involved
|
A number of enzymes involved in the process – 24 Palmitoyltransferase (also known as PATs or DHHCs) and ~15 acyl protein thioesterase (APT) | N-myristoyltransferase | Farnesyltransferase Geranylgeranyltransferase I
Geranylgeranyltransferase II |
|
Motif |
Can occur on cysteines located at different sites in a protein | Occurs only at the N terminus at MGXXXS motif | Occurs only at the CAAX boxes at the C terminus |
|
Example |
eNOS, Arl13b | Src, Cystin | G protein γ subunit, AIPL-1 |
#dissertation #thesis #essay #assignment #phd #phdstudent #phdlife #essaywriting #research #phdjourney #dissertationlife #gradschool #assignments #phdproblems #academicwriting #assignmenthelp #thesiswriting #gradstudent #dissertationproblems #university #academiclife #gradschoolproblems #academia #researchpaper #gradschoollife #graduateschool #college #doctoralstudent #dissertationdone #writing





